Careers
Join our Team
We know that for individuals with rare muscle disorders, every day without an effective treatment is a day too late. We are driven by this urgency to develop novel precision medicines for severe and rare disorders. With our drug discovery platform, and our combined expertise in muscle physiology and small molecule drug design, we are working to change the future. Join a passionate team driven to develop innovative therapies for progressive muscle disorders.
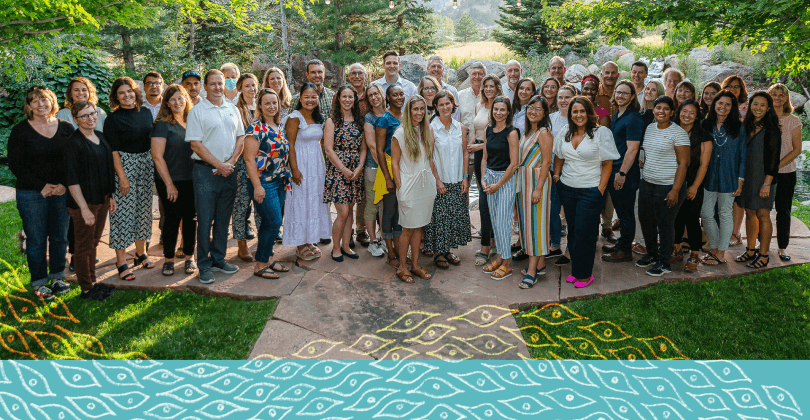

About Us
Our headquarters is in beautiful Boulder, Colorado. While our team operates globally, we all share the same mission.
Our
Values
Our ability to succeed depends on a cross-functional team embracing our core values.


Connection
We connect with patients and families to better understand their experience and needs which help guide our plans. We connect with our team to foster our unique skills and abilities. We treat each other with respect and embrace diversity.

EXCELLENCE
We are intensely focused on our mission to invent medicines that truly make a difference for patients. We excel within our core competencies and understand the value our role brings to the team. We leverage the collective knowledge and skills of our team and external experts to achieve our goals.

COURAGE
We are charting a new course. We learn from the patients we serve – their tenacity and courage fuels our passion. We are driven by their urgent need for innovative therapies. We support final decisions and move forward as a team. We celebrate successes, embrace failures and continuously learn.

Marc Evanchik

"I am proud to work for Edgewise where the passion for good science aligns with the urgency to meet the needs of the currently underserved Duchenne and Becker muscular dystrophy community. I am excited to be part of the team that will bring novel treatment options to these patients."
Maria Mancini
Vice President, Clinical Development Operations

"I joined Edgewise as a start-up and we rapidly invented our lead compound for the treatment of rare muscle disorders. I’m excited to see the growth and success of our company as well as our lead compound, with the hopes that we can make a lasting, positive impact for people with muscular dystrophy."
Ying Qian
Research Investigator, Pharmacology

"I am inspired by the Edgewise focus on the rare diseases of Duchenne and Becker muscular dystrophy. I come to work each day knowing that I can positively impact the quality of the investigational product going to our participants now and the patient population in the future."
Delia Snyder
Associate Director, Quality Assurance

"I feel lucky to be part of this team, this commitment and this science. It's incredible to know that every day we are one step closer to potentially improving the lives of those affected by Duchenne and Becker muscular dystrophy."
Liz Thaler
Director, Clinical Development Operations
Our Team
We are very proud of the Edgewise team and value celebrating our achievements. While our team operates globally, we enjoy seeing each other in person.
Our Benefits
We are proud to offer excellent benefits to our team.

Health Benefits
We provide comprehensive medical, dental and vision benefits. Short-term and long-term disability, life and AD&D insurance is provided as well.
Financial Benefits
We offer an excellent compensation package including competitive base salary, discretionary bonus, opportunities for company ownership (via stock option grants and an employee stock purchase plan) and a 401(k) with match.


Time Away
We want our employees to embrace their lives fully. Our time off plan ensures you have time to get away and recharge.
Current Openings
Become a member of our team. Let’s make an impact together. If you are interested in an open position, please click the link to apply. We look forward to hearing from you.
Edgewise is proud to be an equal opportunity employer. All employment offers are contingent upon the applicant successfully completing a background screen.
Recruiters: Edgewise does not accept resumes from recruitment agencies for our positions. Please do not send resumes to Edgewise employees or the company location. Edgewise is not responsible for any fees related to unsolicited resumes.

