Edgewise’s Approach to Protecting Muscle

We have designed molecules that selectively reduce muscle stress in fast skeletal muscle fibers which are more prone to injury in both Duchenne muscular dystrophy (Duchenne) and Becker muscular dystrophy (Becker).
Our lead molecule, EDG-5506, reduces stress by selectively targeting the muscle motor protein myosin that regulates contraction of fast skeletal muscle fibers. The tissue specificity of EDG-5506 means that the function of other muscles such as slow skeletal, smooth and cardiac muscles are not affected. Allowing fast fibers to contract without injury should preserve muscle health in Duchenne and Becker and potentially enhance physical function.
How does muscle break down in muscular dystrophy?
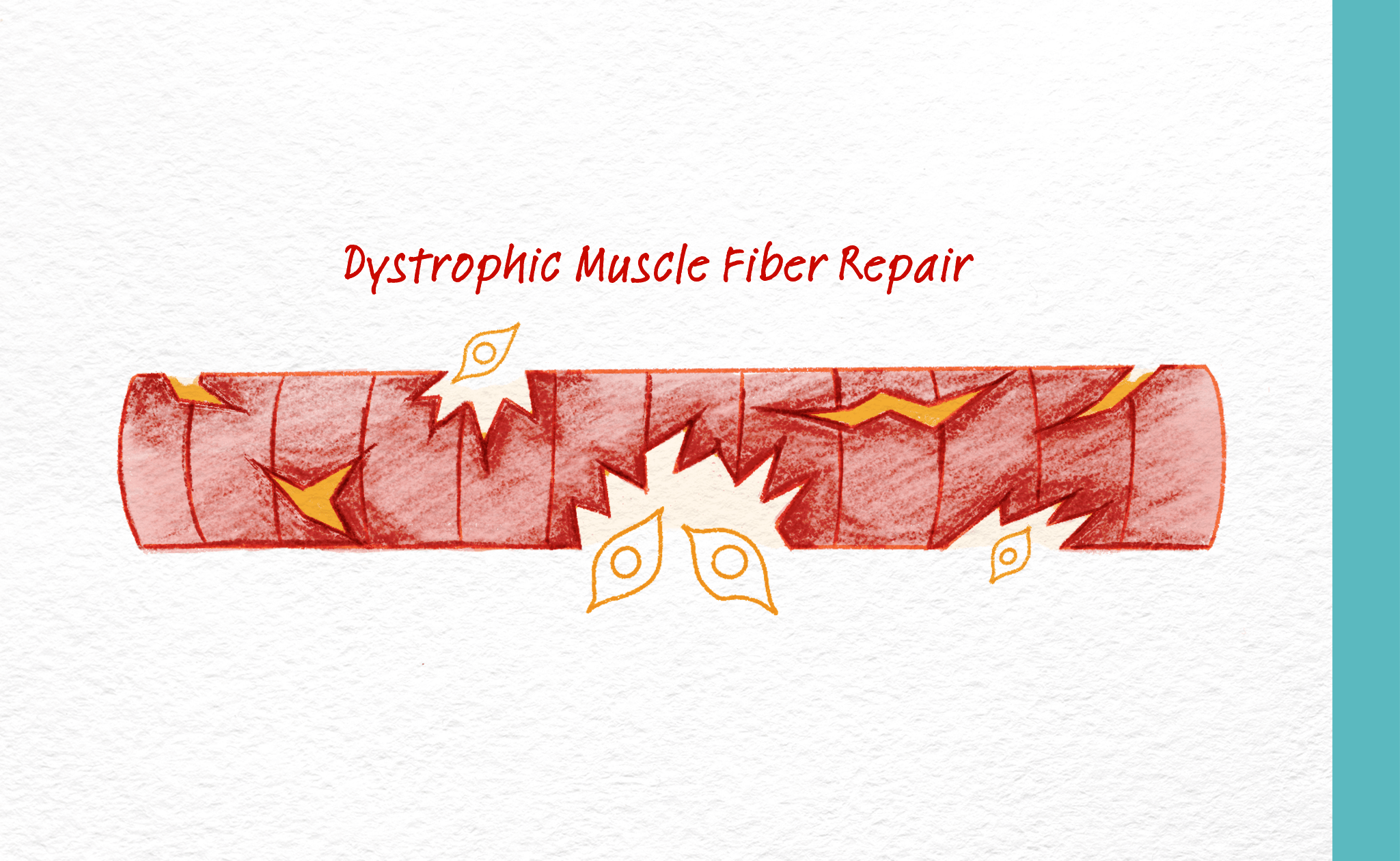
Skeletal muscle is made of parallel bundles of fibers that control movement through contraction. At the outside surface of skeletal muscle is a complex of proteins that includes dystrophin, a large structural protein that acts as a connector between the outside and inside of the muscle. In healthy muscle, dystrophin is one of a group of proteins that work together to protect muscle fibers from injury when muscles contract. In Duchenne and Becker, dystrophin is absent or mutated. As a result, muscle contractions during normal, daily activity leads to excessive muscle injury. When these muscle fibers contract, stress builds up at the muscle membrane which causes fibers to degrade. Normally, muscle fibers regenerate through the activation of stem cells, but in individuals with muscular dystrophy, the rate of new muscle fiber formation can’t keep up with the rate of muscle fiber collapse. The net result is an increase in fibrotic and fatty tissues, in place of healthy muscle.
Why are skeletal muscle fiber types important in Duchenne and Becker?
Humans have two different types of skeletal muscle fibers, termed slow and fast, in a roughly equal mix. As their names suggest, slow fibers tend to contract slower and fast fibers contract faster. Slow and fast fibers work together to coordinate our everyday actions from low levels of activity, all the way to high power activities like sprinting. Research indicates that in Duchenne and Becker, fast fibers are exposed to more physical stress and are more susceptible to injury versus slow fibers.
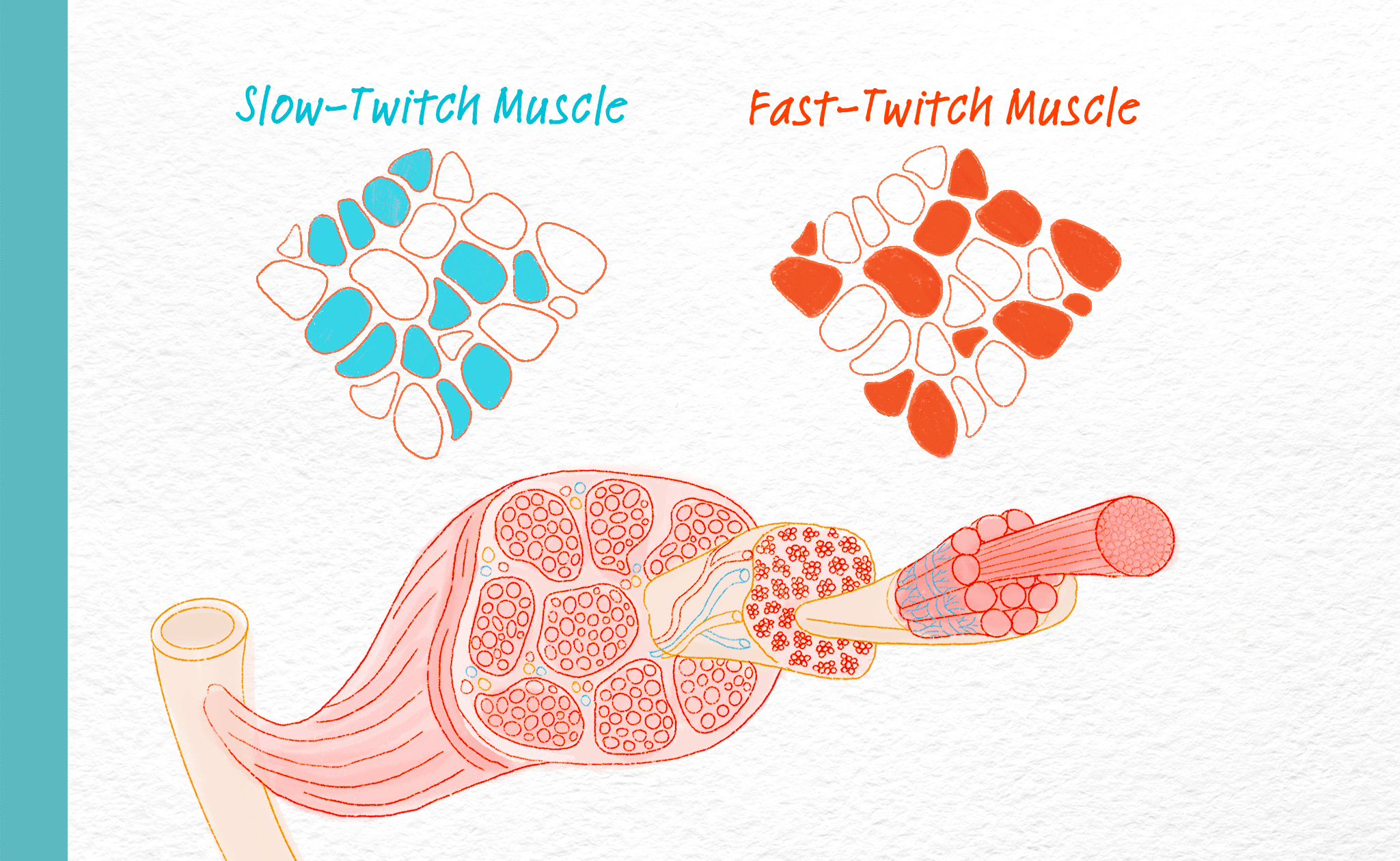
Why does myosin matter in Duchenne and Becker?
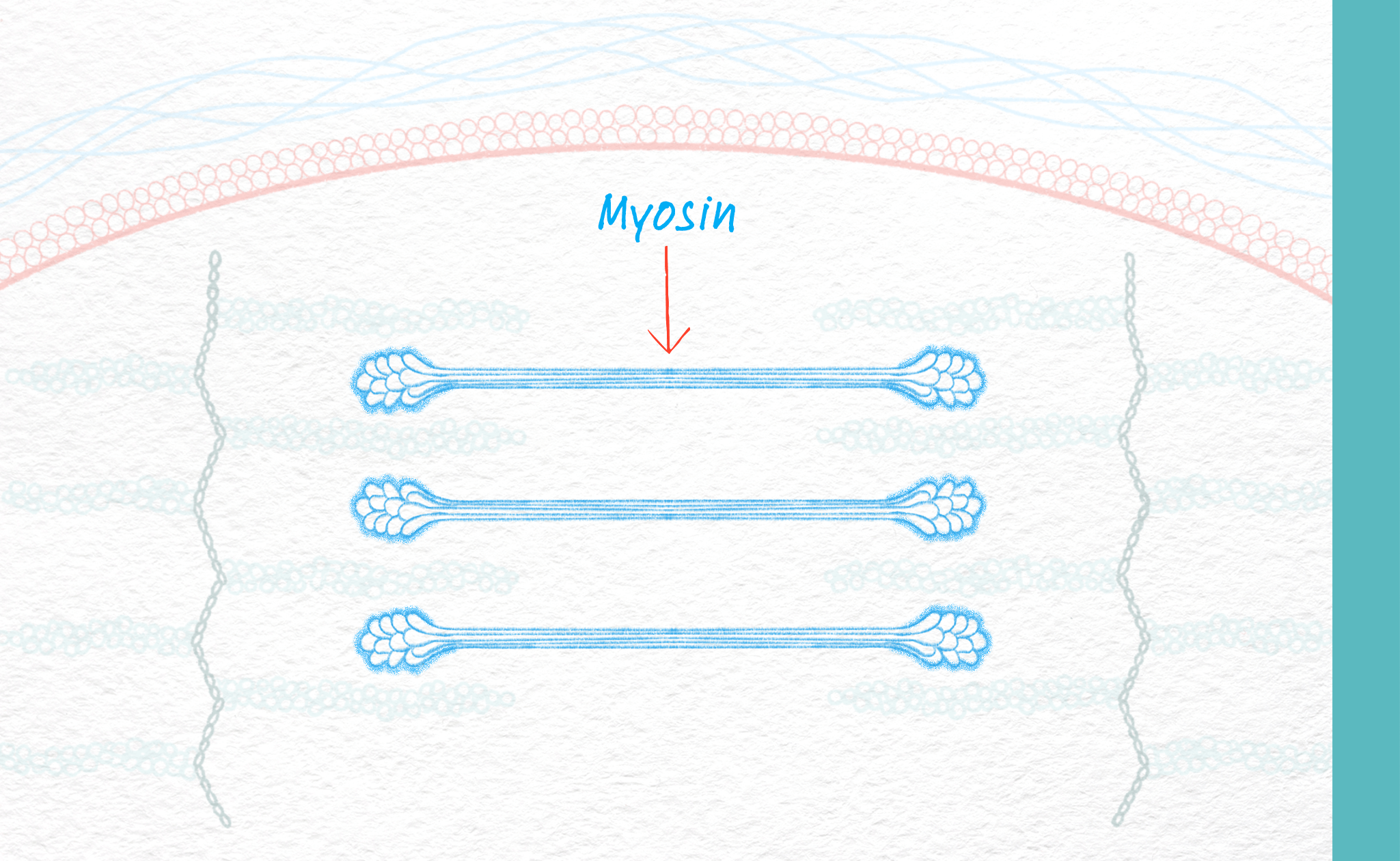
Muscle contraction is key to the stress that causes injury in individuals with Duchenne and Becker. This contraction is driven by a protein called myosin. In Duchenne and Becker, the more force placed on a fiber, especially fast muscle fibers, the more stress that develops, and eventually leads to muscle damage. Individuals with Duchenne and Becker can have relatively normal muscle function even with no or aberrant dystrophin for several years after birth. However, progressive muscle damage and loss ultimately leads to decreases in physical function.
What other muscle disorders is Edgewise considering for EDG-5506?
EDG-5506 has the potential to provide benefit to a broad range of patients where the contraction of skeletal muscle is connected to its degeneration. Beyond Duchenne and Becker, we are also exploring EDG-5506’s potential in several types of limb-girdle muscular dystrophy and metabolic myopathy such as McArdle disease.